The Behavior of Water in Cementitious Hydration with the Addition of Colloidal Nano Silica
By Jon S. Belkowitz, PhD, PE
ABSTRACT
Water remains the primary constituent to initiate the hydration process for concrete strength and increase the workability of concrete for placement. A number of scientists have published research on minimum water content to reach complete cement hydration. Anything over the minimum water content is water of convenience – normally used to increase the workability of the cement composite. However, excess water for workability can also be the initiator of chemical degradation. This research concentrates on understanding the behavior of water in the cement composite and how it can be influenced and manipulated by the introduction of colloidal nano silica particles. Specifically, the impact of three separate colloidal nano silica particles in cement composite samples is demonstrated. What makes this research unique is the analysis of colloidal Nano Silica size on the behavior of water in a cement composite.
Keywords: cement; durability; colloidal nano silica; pozzolan; pore solution
1. INTRODUCTION
Concrete is a composite material, which is made from the combination of water, cement and rock. Over time, the once-fluid concrete hydrates and solidifies into a rock-like form. The hydrated cement matrix is the binder that contributes to the strength and durability of concrete [1-9]. The purpose of this research and series is to investigate and understand how the size of colloidal nano silica influence the behavior in water in a hydrated cementitious composite. Specifically, the role of water and how it affects phase development and strength gain was analyzed and is presented.
How Water Effects Concrete Strength
The cementitious component of the concrete mixture reacts with water to form a matrix of ceramic-like structures that is the back-bone of concrete strength, the calcium-silicate-hydrate (C-S-H). Figure 1 illustrates a schematic of the C-S-H sheets that stack to form the hydrated cementitious matrix (HCM). The C-S-H network relies on an interplay between brittle Ca-O layers and bridging and pairing silicon tetrahedron to form the binding component of the concrete [3,4,5]. Between the C-S-H sheets is interlayer water governing the density and strength of the C-S-H networks [3-5,10].
The center image of Figure 1 shows the interface between a high density C-S-H (left) and low density C-S-H (right) [6]. The extreme left and right images of Figure 1 are magnifications of the C-S-H sheets that make up the larger C-S-H structures and HCM of concrete. Manzano et al recognized the tendency for this lower density C-S-H (OP) to yield softer structures [3]. The cause for this softening of the C-S-H network was attributed to an excess amount of water that enters the crystal-like structural. Within the HCM the pore structure, density and constituents must be taken into account when optimizing concrete for strength and durability as the role of percolation has on the micro-structure and micro-properties all the way through the marco-structure and macro-properties. Thus, an analysis of the composition of the pore water structure in the HCM is warranted.
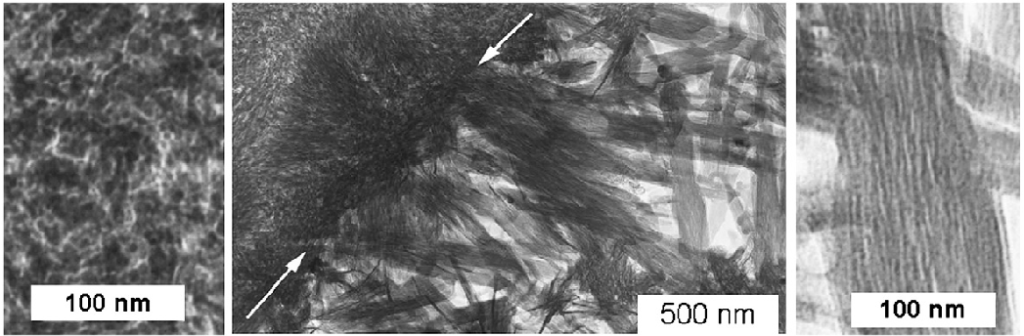
Figure 1. A TEM micrograph showing inner and outer calcium-silicate-hydrates. [6].
Figure 2 illustrates the stages that lead up to the development of pores within the C-S-H network. After water makes contact with the cement particle surface, tri-calcium silicate and di-calcium silicate dissolve and react with the water. As the reactions proceed, calcium hydroxide (CH) precipitates from the cement and water gel. A super-saturated gel of calcium (Ca) and silica (Si) ions is left over after the CH precipitates [8]. As the Ca/Si ratio climbs between 0.8 to 2.3, C-S-H sheets and networks start to develop [3,4,8]. As more C-S-H structures develop, pores grow due to the interaction between adjacent structures and local water content. Figure 2 a represents the different types of water found within the pore structure of the C-S-H network.
Molecular States of Water
Pellenq et al qualify three types of water based on mobility as shown in Figure 2. At the center of the pore is water in its bulk state [6]. Closer to the C-S-H structure, water will start to participate in the cement hydration process. This water has less mobility then the bulk water due to being bound by reactions. Finally, chemically incorporated water has minimal water mobility and is referred to as having a glassy-nature [6]. It has been determined by numerous research groups that as water content is increased past that necessary for hydration (called the critical water-to-cementitious ratio), the size and distribution of pores increase [4,8,11,12]. Ultimately the compressive strength of concrete is affected by the size and density of pores within the concrete composite [4,8], which is related to the water included in the concrete mixture during processing.
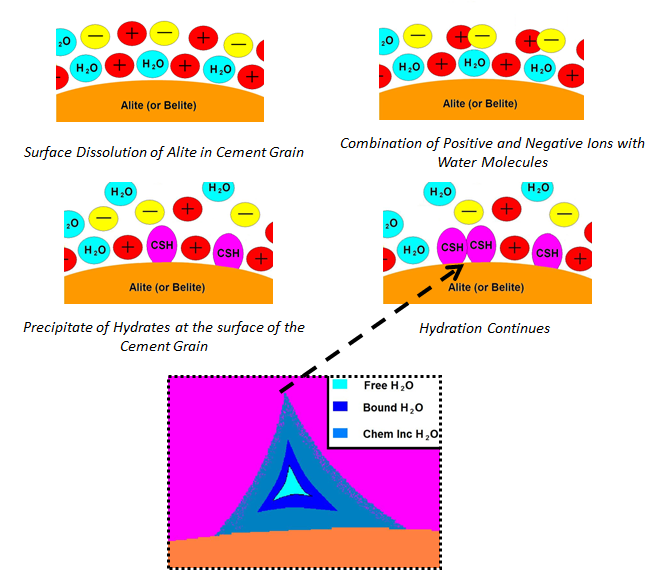
Figure 2. Pore water schematic: the hydrated cement matrix is developed through the dissolution of cement and the reaction with water and ions in the cement paste. As the C-S-H networks start to develop pores are created by adjacently forming C-S-Hs and interactions between other hydrated phases [10, 16].
Stay tuned for our next Blog on How Colloidal nano silica Size Effects Water in the Hydrated Cementitious Matrix of Concrete.
